Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A Case Report on A Patient Presenting with Abdominal Pain Caused by A Large Superior Mesenteric Artery Aneurysm
*Corresponding author:Ranasinghe Leonard, Professor of Emergency Medicine & the Director of M4 Sub-Internships and Electives, California Northstate University College of Medicine, California, USA.
Received:March 10, 2023; Published:March 27, 2023
DOI: 10.34297/AJBSR.2023.18.002462
Abstract
Superior Mesenteric Artery Aneurysms (SMAAs) are a rare type of Visceral Artery Aneurysm (VAA) in the abdomen; aneurysms in this region are most often abdominal aortic aneurysms. In this report, we discuss the case of a 52-year-old male brought in by ambulance for a 3-day history of severe abdominal pain radiating to the back. CT imaging of the abdomen confirmed a superior mesenteric artery aneurysm approximately 7 cm in diameter. He was subsequently hospitalized, and endovascular repair of the aneurysm was performed. We explore the diagnosis, treatment, and outcomes of superior mesenteric artery aneurysms in general and as related to our patient.
Introduction
A true aneurysm is a dilation of all three layers of the blood vessel which include the tunica intima, tunica media, and tunica externa. A pseudoaneurysm, also known as a false aneurysm, is a rupture of the tunica intima and media layers of a blood vessel that usually causes leakage of blood into surrounding tissue. A dissection is a separation within the blood vessel wall due to blood entering through a tear in the intima layer [1]. This case revolves around the superior mesenteric artery, which is a branch of the aorta that supplies the small intestine to the splenic flexure of the large intestine [2]. In addition, visceral artery aneurysms include aneurysms of the celiac, mesenteric, hepatic, splenic, and renal arteries. Therefore, superior mesenteric artery aneurysms are a subset of visceral artery aneurysms.
There are numerous risk factors that can lead to an aneurysm. These risk factors primarily involve some form of injury to the arterial vessel wall. Damage to the vessel wall may range from direct trauma to chronic conditions such as atherosclerosis, in which the high serum levels of cholesterol or glucose damage the elastic fibers of arteries and lead to stiffening [3]. Rarer conditions that involve non-inflammatory, non-atherosclerotic obstructive arterial disease, such as fibromuscular dysplasia, affect mid-size arteries that lead to severe narrowing of the arterial lumen [4]. Connective tissue disorders such as Ehlers-Danlos syndrome may also weaken the collagen fibers in the tunica intima layer of arteries leading to aneurysm and rupture [5]. Furthermore, infection can weaken a vessel wall due to hematogenous spread of septic emboli, causing a mycotic aneurysm. Pathogens can spread to the vasa vasorum of an artery and cause infectious arteritis. Patients at higher risk of bacterial infection, including those with cardiac valvular abnormalities or prosthetic valves, intravenous drug abuse, and immunosuppressed conditions such as HIV infection are more prone to aneurysm development and rupture [6]. In addition, there have been case reports on vasculitis as a potential instigator of visceral artery aneurysms as well. One case report discussed a 67-year-old woman with an ANCA-positive vasculitis of the anti- PR3 subtype who demonstrated multiple visceral abdominal aneurysms, particularly in the inferior and superior mesenteric arteries [7].
Visceral artery aneurysm diagnoses are becoming more common with the implementation of radiological imaging such as ultrasound, Computed Tomography (CT) scan, and Magnetic Resonance Imaging (MRI). However, visceral artery aneurysms are overall rare; in contrast, approximately 95% of all abdominal aneurysm are aortic or renal in nature [8]. Superior mesenteric artery aneurysms, or SMAAs, are even more rare and are estimated at making up 3-9% of all visceral artery aneurysm cases [8]. Due to the rare nature of SMAAs, the understood causes of SMAAs are changing. Previously, an estimated 60% of SMAAs were thought to be due to mycotic causes; however, recent research suggests that vascular wall degeneration, inflammation of surrounding arteries from pancreatitis, and trauma are more likely the cause [8,4]. SMAA rupture is also high at 38-50% with a high chance of mortality, which is the reason immediate surgical intervention is usually indicated for patients instead of monitoring the aneurysm first. SMAA symptoms include but are not limited to severe abdominal pain, nausea, vomiting, bloody bowel movements, constipation, and a pulsating mass in the abdomen. In this case report, the patient presented with several of these nonspecific symptoms which led the care team to first consider abdominal aortic aneurysm.
As SMAAs are rare, abdominal aortic aneurysms are often initially suspected due to its more common occurrence. Abdominal aortic aneurysms, or AAAs, are defined as abdominal aortic dilation of 3cm or greater. AAAs with a dilation of greater than 5cm are indicated for surgery. AAAs have a much higher incidence in males with an estimated incidence of 4-7% in men compared to 1% in women [9]. Other risk factors for AAA include age greater than 65 years old, smoking, hypertension, hypercholesterolemia, obesity, arterial disease such as atherosclerosis and coronary artery disease, history of other vascular aneurysms, as well as a family history of AAAs [10]. Factors that decrease the likelihood of an AAA include African American race, Hispanic ethnicity, and diabetes. The majority of AAAs are asymptomatic and are found incidentally on abdominal imaging such as with ultrasound or CT angiography. Symptoms classically associated with AAAs include abdominal pain with radiation to the back [11].
In this paper, the patient’s case details will be presented along with a discussion of the patient’s rare superior mesenteric artery aneurysm and complications.
Case Details
Patient Details
The patient was a 52-year-old Spanish speaking male who was brought in by ambulance to the emergency room with a three-day history of severe abdominal pain radiating to the back. Patient denied pertinent past medical and surgical history. He had a positive urine test for methamphetamines despite his denial of drug or alcohol use. The patient also reported smoking cigarettes daily, while the pack year history was unknown. The patient was noted to be without permanent housing, and overall, the history was limited due to a language barrier and the patient being a poor historian.
Case Presentation
The patient was brought in by ambulance for severe abdominal pain that started three days prior. The pain was getting progressively worse and was located mainly in the epigastrium with radiation to his back. The patient also felt nauseous and had vomited one day prior but had not vomited on the day of presentation. He did not notice blood in the vomit. The patient denied chest pain or shortness of breath. The initial differential diagnoses upon arrival to the ED included pancreatitis, gastritis, or perforation.
Physical Examination and Other Investigations
The patient was hypertensive in the 180-190s en route to the hospital per the EMS. After arriving at the ED, the patient’s vital signs were taken: temperature of 98.4℉ (36.9℃), heart rate of 65 bpm, respiratory rate of 28 breaths per minute, and blood pressure of 176/94 mmHg. Gastrointestinal examination revealed soft, moderate to severe epigastric tenderness with guarding. The patient’s lungs were clear to auscultation, and his respirations were non-labored upon respiratory examination. Other physical examination results were unremarkable.
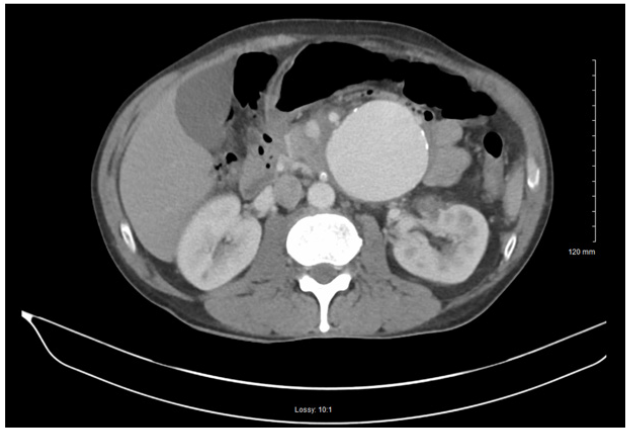
The patient’s chemical panel revealed hyponatremia (131mMol/L, normal range: 135-145mMol/L), hypocapnia (19mMol/L, normal: 23-29mMol/L), hyperglycemia (150mg/dL, normal: 70-105mg/dL), hypocalcemia (4.3mg/dL, normal: 8.5-10.5 mg/dL). The CBC revealed microcytic anemia (normal Hgb: 14-18g/ dL) and lymphocytopenia (normal WBC: 4.5-11k/mcL). In addition, the patient’s liver enzymes (ALT: 59U/L, normal: 4-43U/L; AST: 66U/L, normal: 9-35U/L), were elevated, providing suspicion for alcohol abuse although the patient denied any alcohol or drug use. The patient’s urine drug screen was positive for methamphetamines. The electrocardiogram results were unremarkable with no signs of ST Segment Elevation (STE) or T-Wave Inversion (TWI). The chest x-ray was negative for acute disease. A contrast CT scan was done of the patient’s abdomen and pelvis. The CT revealed a very large aneurysm arising from the left aspect of the superior mesenteric artery measuring approximately 6.7cm x 7.1cm. Inflammation around the aneurysm was seen, indicating infection, vasculitis, or early signs of rupture. In addition, severe stenosis of the origin of the celiac artery was found (Figure 1).
Treatment Plan and Management
The patient was admitted due to his unstable condition and a vascular surgeon was consulted. The patient was given IV fentanyl for pain, IV zofran (ondansetron) for his nausea, and a normal saline bolus in the ED. Inpatient medications included: famotidine, docusate sodium, acetaminophen, and bisacodyl. IV orders included: hydralazine, saline solution, hydromorphone, ondansetron, metoclopramide, and fentanyl.
The patient did not have bowel movement or flatulence for three days, but the patient’s physician noted no evidence of peritonitis or bowel ischemia. This was monitored and addressed with bowel rest and frequent ambulation. To address his hypertension, nitroprusside drip was administered to maintain a systolic blood pressure below 150mmHg. Liver function was continually monitored due to signs of transaminitis, likely due to chronic alcohol use. Magnesium was repleted as needed and the patient’s hyponatremia, hyperglycemia, and anemia were monitored. Protonix was given daily for prophylaxis and a sequential compression device was used for the prevention of deep vein thrombosis.
Surgical Intervention
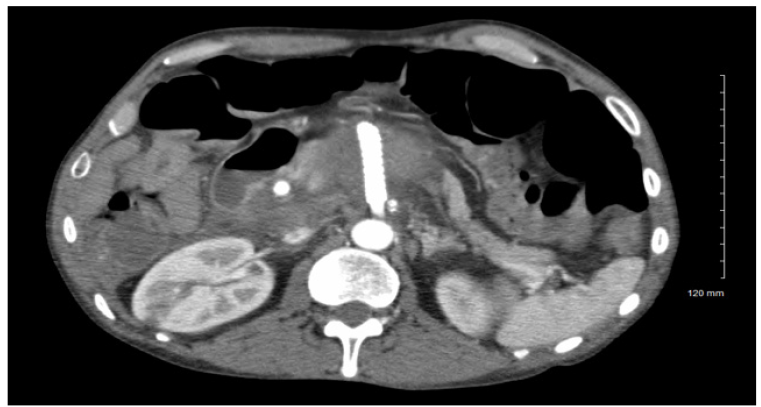
After imaging, the patient was confirmed to have a visceral artery aneurysm in the superior mesenteric artery as well as celiac artery stenosis. Upon consultation with a vascular surgeon, surgical repair of the SMAA was requested. An endovascular approach through femoral access was performed and a GORE® VIABAHN® VBX balloon expandable stent (8mm x 59mm) was deployed in the proximal SMA. Attempts to canalize the celiac artery from the femoral approach were unsuccessful. After the endovascular repair, a fluoroscopy of the aneurysm showed some retrograde filling or a possible bowel artifact. The patient was prescribed clopidogrel and a CT angiography of the aorta with bilateral extremity runoffs was ordered to assess postoperative aneurysm changes. The patient appeared stable and was moved from the ICU to a hospitalist service. With the unsuccessful canalization of the celiac artery from a femoral approach, the patient was scheduled for a brachial artery approach (Figure 2).
The recanalization of the celiac artery was followed by a covered VBX balloon expandable stent (6mm x 19mm), coil embolization of the distal pancreaticoduodenal artery outflow from the SMAA, and coil embolization of the proximal jejunal artery supplying the SMAA. Static contrast following the embolization looked promising for aneurysm thrombosis, and collateral flow from another jejunal branch supplied the territory of the recent embolization, lowering concern for bowel ischemia. The patient was then prescribed clopidogrel, heparin, amlodipine, and lisinopril to reduce clotting risk and help control the patient’s blood pressure.
Post operation, the patient’s arm became swollen at the brachial access point and a hematoma with tracking up the arm was found during an ultrasound. The patient denied any arm pain or numbness but did complain of epigastric pain. A pressure dressing was placed on the arm at the site of swelling. The patient was monitored for bowel ischemia and was placed on a clear liquid diet in case of ileus. A follow up CT angiography was performed on the patient to check for ileus and to reexamine the SMAA. Based on imaging, internal flow of the SMAA had been successfully stopped by the coil embolization procedure and the patient was not found to have ileus, so the physician recommended advancing the patient’s diet.
The following day, the patient reported still having some epigastric pain, and he passed gas but had no bowel movements. The patient was anemic with hemoglobin of 7g/dL, hematocrit of 21.1%, mean corpuscular volume of 78.9fL, and mean corpuscular hemoglobin of 26.3pg/cell. Physician recommendation was to have the hemoglobin raised to 8g/dL through infusions. Red cell distribution width was high with Coefficient of Variation (CV) at 19% and Standard Deviation (SD) at 55.3 fL. The patient was also hyponatremic (125mMol/L), hypochloremia (93mMol/L, normal: 95-105mMol/L), hypo uremic (BUN 6mg/dL, normal: 7-18 mg/ dL), and hypocalcemia (8.2mg/dL). Two days after CT angiography, the patient’s epigastric pain had improved with mild tenderness on palpation. Hemoglobin was raised to 10.3 g/dL and the patient was tolerating PO intake.
Discussion
Causes and Risk Factors
One of the possible causes for superior mesenteric artery aneurysm is infection. Bacterial infection is thought to be a less common cause of SMAAs compared to other causes including vascular wall degeneration, surrounding organ inflammation, or trauma. In this case, infection was considered as a possible cause of the patient’s SMAA due to his methamphetamine use [8]. Symptoms of mycotic aneurysm of the superior mesenteric artery include abdominal pain, low grade fever, malaise, weight loss, and nausea and vomiting. This patient had severe epigastric pain as well as nausea and vomiting but no fever and lymphocytopenia. However, only 60% of mycotic SMAA cases in a recent literature review presented with low-grade fever [12]. Besides intravenous drug use, septic embolization from subacute endocarditis was found to be another common cause of mycotic SMAAs. There were also single case instances found of mycotic SMAAs from suppurative hidradenitis, infected heart device implants, and rheumatic heart disease [12]. Recent literature points to Streptococcus viridans as the most causal organism of SMAA and inferior mesenteric artery aneurysms, with the next most likely to be Staphylococcus aureus [12,13]. Furthermore, conditions that weaken the tunica intima layer of blood vessels, including vascular wall degeneration and atherosclerosis, can predispose people to infection from Staphylococcus and Streptococcus, compounding the risk of a SMAA [12].
In the case of this patient, some of the major risk factors leading to the development of an SMAA were likely the use of methamphetamines and uncontrolled hypertension leading to atherosclerosis. Atherosclerosis is a risk factor for SMAAs and other VAAs, however it has been shown to possibly play either a primary or secondary role in the development of these aneurysms [14,15]. Methamphetamine use can lead to atherosclerosis through multiple proinflammatory pathways. In a study on mice, methamphetamine use was shown to increase endothelial expression of intercellular adhesion molecule-1, vascular adhesion molecule-1, and monocyte chemoattractant-1 [16]. Furthermore, methamphetamine use elicited a proinflammatory response from macrophages through noticeably increased production of ROS, IL-6, and IL-1 beta, all of which are atherogenic factors [16]. These atherogenic cytokines and chemokines can induce macrophages to release MMP-2 and MMP-9, which are metalloproteinases that can contribute to arterial wall degradation and aneurysm dilation [17].
Imaging and Diagnoses
Although initial suspicion of an SMAA can be raised based on an abdominal ultrasound examination, the presence of an SMAA can be more precisely detected using CT angiography or MRI. On duplex ultrasound of an aneurysm, turbulent flow can be noted by observing spectral broadening waveforms which reflect heterogeneous velocities of red blood cells [18,19]. There has been contention on the use of digital subtraction angiography (DSA) over CT angiography for diagnosing aneurysms; however, recent studies have demonstrated CT angiography is capable of diagnosing small aneurysms less than 3 cm with DSA as the reference standard [20]. In addition, the high cost and length of DSA have limited its widespread use in detecting aneurysms despite its high diagnostic value [21]. In this particular patient case, emergent mesenteric CT angiography was performed on the patient and a 6-7 cm saccular aneurysm was visualized upon imaging. Magnetic resonance angiography (MRA) is another powerful tool for detecting aneurysms, and compared to CT angiography, it lacks ionizing radiation and uses less toxic contrast dyes. It is an especially robust technique in vascular systems because of reduced T1 relaxation times and therefore higher contrast resolution [22]. Multi-Detector Computed Tomography (MDCT) can yield a highly accurate representation of SMA course and caliber so that the diameter of the aneurysm can be measured [23]. Aneurysm diameter is a key element in determining optimal treatment. Smaller visceral artery aneurysms, those less than 2 cm in diameter, are sometimes subject to “watchful waiting” and checked periodically for any signs of growth. In addition, calcified arterial walls on x-rays might raise the suspicion of an aneurysm [24]. Coupled with abnormal changes in the surrounding organs and tissues, such as signs of chronic pancreatitis along with stomach or flank pain, suggest the presence of a VAA.
Management Approaches and Outcomes
While most VAAs are asymptomatic, symptomatic VAAs are at high risk for rupture and should be treated as soon as possible [14]. SMAAs have been found to be more symptomatic than the other types of VAAs with symptoms that include vomiting, abdominal angina, gastrointestinal bleeding, and epigastralgia. SMAA ruptures are dangerous with a rate of rupture estimated between 38-50% and an associated mortality of 30-90% [8,25]. The high mortality rate is related to intestinal ischemia sometimes caused by the rupture. Specifically, thrombosis from the rupture can lead to intestinal hypoperfusion, which can lead to ischemia and eventual death [8,14].
There are many approaches to the management of SMAAs and VAAs, including open surgery and endovascular therapy. Endo Vascular Therapy (EVT), like embolization, is minimally invasive and can be an effective approach for high-risk patients with comorbidities. Another option, open repair, has been shown to be effective in allowing surgeons to visualize the necessity of vascularization during surgery, potentially decreasing the need for a second operation. Despite these benefits, the indications for EVT have expanded, making it a potentially more effective approach. This has led to open surgery being more selected as an option when EVT is too difficult to perform [8,25,2]. Selection of the specific treatment approach depends on a variety of factors including location and size of the aneurysm, available resources, and individual preference of the surgeon. Other associated symptoms presenting alongside SMAAs will affect management selection as well. Examples include septic emboli, pancreatitis, biliary tract disease, polyarteritis nodosa, atherosclerosis, trauma, and fibromuscular dysplasia [14].
Unfortunately, few studies compare the efficacy of open surgery versus an endovascular approach regarding VAAs and SMAAs. However, a recent review of VAA management suggests that an endovascular approach was associated with a shorter hospital stay and lower rates of cardiovascular complications [26]. No significant differences in mortality rates were found when comparing open surgery and endovascular therapy, but reintervention rates were higher after endovascular therapy compared to open surgery. However, procedural complications were more common with an open approach than with EVT. Some complications listed included wound infection and respiratory complications. For these reasons, the review suggested that EVT could be recommended as being a first choice to treat VAAs [26]. Another study examined 10 patients with SMAAs, where 5 patients underwent EVT and 2 patients underwent open surgery. The mean operation time for open surgery was about 3.6 hours while the mean operation time for endovascular treatment was about 1.3 hours. The mean hospital stay after the operation was 20 days in the open surgery group and 2.2 days in the endovascular group. Although the sample size is limited, this further suggests the benefits of an endovascular approach with an associated shorter operation and hospital stay [25].
Similar to the results found in regard to VAAs, in a study on AAA repair, there was no significant difference in the long-term mortality in the patients with either an endovascular or open approach. However, there were more secondary procedures needed in patients that underwent the endovascular repair which was also seen with VAAs [26,3].
Conclusion
This case contributes to the body of knowledge surrounding SMAAs due to the rarity of SMAAs and the circumstances of the patient. This patient had many potential health risk factors, including homelessness and positive screening for methamphetamine use, that could have contributed to the inflammatory process seen on CT imaging. The patient also had a relatively large SMAA at 6.7 x 7.1cm and the endovascular repair used in this case is still being documented for its efficacy in relation to open repair surgeries. This patient’s surgical complications and need for a secondary intervention follow the trend seen in other studies of endovascular repair of VAAs and AAAs [26,3].
Acknowledgement
None.
Conflict of Interest
None.
References
- Kemp WL, Burns DK, Brown TG (2008) Pathology: The Big Picture, McGraw Hill.
- Shaikh H, Wehrle CJ, Khorasani Zadeh A (2022) Anatomy, Abdomen and Pelvis, Superior Mesenteric Artery. In: StatPearls. Treasure Island (FL): StatPearls Publishing.
- Frank A Lederle, Tassos C Kyriakides, Kevin T Stroupe, Julie A Freischlag, Frank T Padberg, et al. (2019) Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med 80(22): 2126-2135.
- Friedman SG, Pogo GJ, Moccio CG (1987) Mycotic aneurysm of the superior mesenteric artery. J Vasc Surg 6(1): 87-90.
- Atsuo Kojima, Shunya Shindo, Kenji Kubota, Keiji Iyori, Tadao Ishimoto, et al. (2002) Successful surgical treatment of a patient with multiple visceral artery aneurysms due to fibromuscular dysplasia. Cardiovasc Surg 10(2): 157-160.
- Eser Adiguzel, Pamela J Ahmad, Christopher Franco, Michelle P Bendeck (2009) Collagens in the progression and complications of atherosclerosis. Vasc Med 14(1): 73-89.
- Abissegue Y, Lyazidi Y, Arache W, Ouldsalek E, Chtata HT, Taberkant M (2016) Multiple Visceral Artery Aneurysms: An Uncommon Manifestation of Antineutrophil Cytoplasmic Antibody Vasculitis. Ann Vasc Surg 34: 271.e9-271.e13.
- Obara H, Kentaro M, Inoue M, Kitagawa Y (2020) Current management strategies for visceral artery aneurysms: an overview Surg Today 50(1): 38-49.
- Michael LL Fevre, US Preventive Services Task Force (2014) Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 161(4): 281-290.
- Keisler B, Carter C (2015) Abdominal aortic aneurysm. Am Fam Physician. 91(8): 538-543.
- Ullery BW, Hallett RL, Fleischmann D (2018) Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom Radiol (NY) 43(5): 1032-1043.
- Kordzadeh A, Watson J, Panayiotopolous YP (2016) Mycotic aneurysm of the superior and inferior mesenteric artery. J of Vascular Surgery 63(6): 1638-1646.
- Stone WM, Abbas M, Cherry KJ, Fowl RJ, Gloviczki P (2002) Superior mesenteric artery aneurysms: is presence an indication for intervention? J Vasc Surg 36(2): 234-237.
- Fady Ibrahim, Jonathan Dunn, John Rundback, John Pellerito, Andrew Galmer (2018) Visceral Artery Aneurysms: Diagnosis, Surveillance, and Treatment. Curr Treat Options Cardiovasc Med 20(12): 97.
- Gehlen JMLG, Heeren PAM, Verhagen PF, Peppelenbosch AG (2011) Visceral Artery Aneurysms. Vascular and Endovascular Surgery 45(8): 681-687.
- Christopher G Kevil, Nicholas E Goeders, Matthew D Woolard, Shenuarin Bhuiyan, Paari Dominic, et al. (2019) Methamphetamine Use and Cardiovascular Disease. Arterioscler Thromb Vasc Biol 39(9): 1739-1746.
- Peshkova IO, Schaefer G, Koltsova EK, (2016) Atherosclerosis and aortic aneurysm - is inflammation a common denominator?. FEBS J 283(9):1636-1652.
- Byers PH. Vascular Ehlers-Danlos Syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al. (1999) eds. GeneReviews®. Seattle (WA): University of Washington, Seattle: September 2.
- Xuedong Xu, Alicia L Eubanks, Alan Wladis, Paula Veldhuis, Steve Eubanks, (2019) Mycotic Superior Mesenteric Artery Aneurysm: Case Report and Literature Review. Surg Innov 26(2): 260-264.
- Zhen Lu Yang, Qian Qian Ni, U Joseph Schoepf, Carlo N De Cecco, Han Lin, et al. (2017) Small Intracranial Aneurysms: Diagnostic Accuracy of CT Angiography. Radiology 285(3): 941-952.
- Xiaodan Chen, Yun Liu, Huazhang Tong, Yonghai Dong, et al. (2018) Meta-analysis of computed tomography angiography versus magnetic resonance angiography for intracranial aneurysm. Medicine (Baltimore) 97(20): e10771.
- Massimo Venturini, Filippo Piacentino, Andrea Coppola, Valeria Bettoni, Edoardo Macchi, et al. (2021) Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives. J Clin Med 10(11): 2520.
- Stefano Palmucci, Letizia Antonella Mauro, Pietro Milone, Francesco Di Stefano, Antonino Scolaro, et al. (2010) Diagnosis of ruptured superior mesenteric artery aneurysm mimicking a pancreatic mass. World J Gastroenterol 6(18): 2298-2301.
- Lovro Tkalčić, Berislav Budiselić, Miljenko Kovačević, Siniša Knežević, Slavica Kovačić, et al. (2017) Endovascular Management of Superior Mesenteric Artery (SMA) Aneurysm - Adequate Access is Essential for Success - Case Report. Pol J Radiol 82: 379-383.
- Jianjun Jiang, Xiangjiu Ding, Qingbo Su, Guangyong Zhang, Qingliang Wang, et al. (2011) Therapeutic management of superior mesenteric artery aneurysms. J Vasc Surg 53(6): 1619-1624.
- Patricia Barrionuevo, Mahmoud B Malas, Besma Nejim, Abdullah Haddad, Allison Morrow, et al. (2019) A systematic review and meta-analysis of the management of visceral artery aneurysms. Journal of Vascular Surgery 70(5): 1694-1699.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.